One of the key innovations that arose in vertebrates was the evolution of a branch of the nervous system called the sympathetic nervous system. This forms from cells produced by stem cells that originate in a structure called the neural crest. The sympathetic nervous system is associated with the fight-or-flight response and the neurotransmitter signalling molecules adrenaline and noradrenaline. Writing in Nature, Edens et al.1 report that this system is not found solely in jawed vertebrates, and that the basic building blocks and developmental regulators of a sympathetic nervous system are also present in the jawless vertebrate fish the sea lamprey (Petromyzon marinus).
Read the paper: Neural crest origin of sympathetic neurons at the dawn of vertebrates
This discovery refines the understanding of the nervous system and how it functions in a vertebrate closely related to the earliest known vertebrates. This landmark study uncovers a characteristic molecular and developmental ‘fingerprint’ present in noradrenaline-producing (noradrenergic) sympathetic neurons in sea lampreys that is found across many classes of vertebrates, from jawless fishes to mammals (Fig. 1). Studies from the nineteenth and into the twentieth century culminated in the shared opinion that the jawless fishes are not equipped with an ‘organized’ sympathetic nervous system2 — represented by structural hallmarks such as a chain of clustered neurons called sympathetic ganglia.
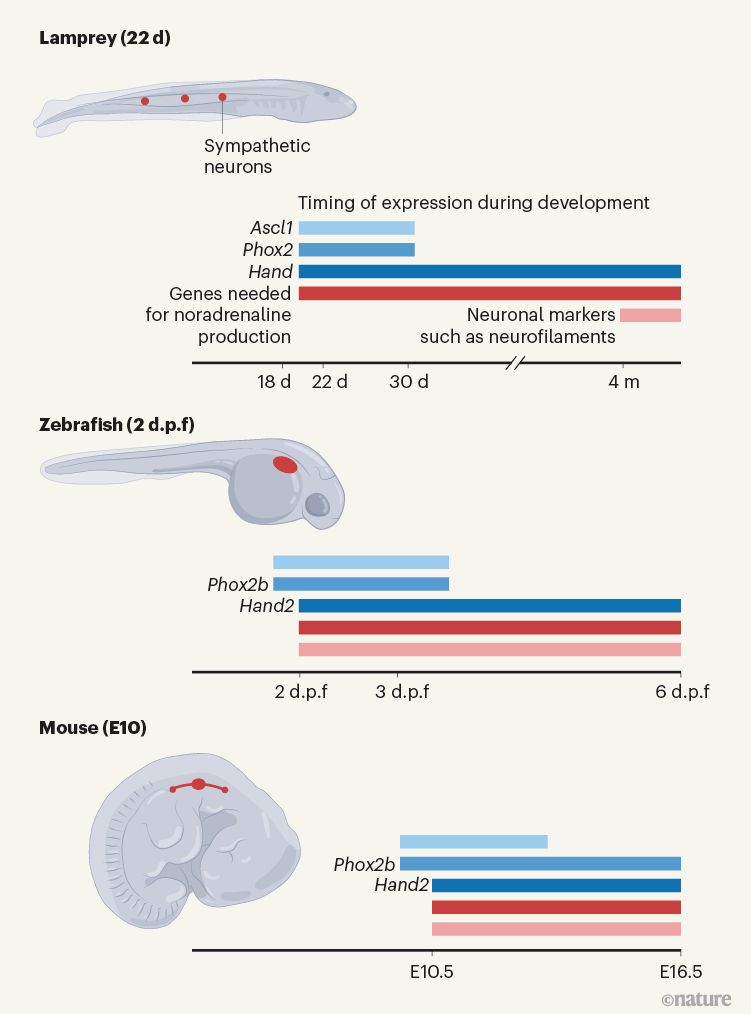
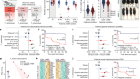
Figure 1 | Sympathetic neurons in different types of vertebrate. Edens et al.1 report that these neurons (associated with the fight-or-flight response) are not found solely in jawed vertebrates but are also found in the jawless vertebrate sea lampreys (Petromyzon marinus). Red indicates the sites in the developing embryos at which sympathetic neurons first differentiate. Genes encoding the transcription factor proteins Ascl1, Phox2 (Phox2b in jawed vertebrates) and Hand (Hand2 in jawed vertebrates) are needed for sympathetic neurons to develop. When these neurons form, they express enzymes (such as tyrosine hydroxylase) that produce the neurotransmitter molecule noradrenaline. These neurons also express components (such as the protein synaptotagmin 1), which function in the neurotransmitter-containing vesicles. In sea lampreys, the neuronal differentiation associated with the production of characteristic neuronal components such as structures called neurofilaments occurs relatively late in development compared with the case of jawed vertebrates, such as zebrafish (Danio rerio) or mice. Data from refs 1, 4, 5, 7, 8 and 11. Sea lampreys hatch after 10—13 days of embryonic development and form larvae at 33—40 days. Zebrafish hatch after 48 hours and become early larvae at 72 hours. They reach juvenile stages by 30—40 days and reach sexual maturity within 2.5 months. Mice reach a mid-embryonic stage by 10 days, are born at 19 to 21 days and reach sexual maturity by around 40 days. D, day of development; E, day of embryonic development; d.p.f., days post-fertilization; m, months.
However, the availability of methods to explore gene expression has provided data that revises the old view and establishes the sympathetic nervous system as a pan-vertebrate feature. Edens and colleagues’ work completes the search for the evolutionary onset of the principal components of the vertebrate autonomic nervous system — the part of the nervous system (including the sympathetic nervous system) that regulates involuntary processes that are essential for survival. This study marks a milestone in this field of research, the implications of which range from understanding the generation of cell identities across evolutionary time scales to the development of anticancer therapeutics in accessible model organisms.
Edens et al. present a detailed picture of the developmental expression of key transcription factors and functional signature genes that are characteristic of sympathetic neurons. The method of in situ hybridization chain reaction reveals that transcripts encoding Ascl1, Phox2 and Hand, related to three evolutionarily conserved transcription factor proteins, Ascl1, Phox2b and Hand2 (which are known to be important for sympathetic neuron development in jawed vertebrates) are co-expressed in the same cells in the sea lamprey. These transcription factors were also co-expressed with genes that encode the enzymes tyrosine hydroxylase and dopamine β-hydroxylase, which are involved in the synthesis of noradrenaline.
Cell tracing by dye labelling revealed that the cells that express tyrosine hydroxylase are derived from a migratory population of cells that are initially located in the spinal cord and that differentiate into a nerve cord between the intestine and a structure called the notochord. RNA sequencing of cells that express tyrosine hydroxylase in the nerve cord demonstrated a gene-expression pattern remarkably similar to the pattern found in mouse sympathetic neurons3. The genes encoding tyrosine hydroxylase, dopamine β-hydroxylase and the enzyme DOPA decarboxylase (which is also involved in the synthesis of noradrenaline) were found to be highly expressed with several genes encoding proteins that function in vesicles that are associated with synapses (connections between neurons). These vesicular proteins include vesicular monoamine transporter 2, which packages neurotransmitter molecules into vesicles, and the proteins synaptotagmin 1, SNAP-25 and syntaxin 12 — essential for the fusion of vesicles with the cell membrane that enables the regulated release of stored neurotransmitter. Components characteristic of neurons, such as neurofilaments, were detected in mature neurons.
On the crest of becoming vertebrate
The developmental transition that occurs when neural-crest-derived progenitor cells mature to differentiated neurons is still not fully understood. In mice and birds, it is a rapid process in which the initial noradrenergic and neuronal differentiation occur in parallel within one day of embryonic development (Fig. 1). However, the time course of neuronal differentiation in sea lampreys is surprisingly slow by comparison, and has similarities to the delayed generation and asynchronous differentiation of structural components of the sympathetic nervous sysyem (trunk sympathetic ganglia) in zebrafish (Danio rerio)4. Phox2b has an essential role in the differentiation process of structures such as the trunk sympathetic ganglia. This transcription factor is a key regulator of autonomic neuron development in mice, chick and zebrafish5–7, and it controls the expression of a range of neurotransmitter-specific and general neuronal characteristics. Hand2 selectively affects noradrenergic differentiation in zebrafish8 and it is possible that Hand is implicated in this function in sea lampreys. This evolutionary conservation of developmental processes might offer a unique opportunity to study a type of tumour called a neuroblastoma using the zebrafish as a model organism9.
Comparative analysis of sympathetic neuron activity in response to different stimulation settings has firmly established the existence of sympathetic pathways that are specific to different tissues in different organs of the body and the differential control of activity outflow to diverse targets10. Developmental studies and single-cell analyses of gene expression in mice have identified a range of sympathetic neuron populations that are associated with distinct cardiovascular, thermoregulatory and maternal functions. These have different gene-expression patterns and distinct requirements for growth-factor proteins during development3.
Edens and colleagues’ observation of the co-expression of the gene encoding tyrosine hydroxylase and the gene encoding the neuropeptide NPY in sea lamprey sympathetic neurons provides evidence for the existence of a neuron population that is comparable with that of an abundant noradrenergic population in mice, called NA3. This population is characterized by NPY expression that is representative of vasoconstrictor neurons that regulate blood-vessel diameter and resistance. The authors’ discoveries point to a remarkable diversification of sympathetic neuron populations among vertebrate classes and species. The findings also prompt questions about the selective pressures and genomic mechanisms that underlie this evolutionary outcome across the tree of life, such as those that lead to the crucial involvement of the sympathetic nervous system in thermoregulation in birds and mammals.
One key unanswered question in the current study is whether the neurons with key noradrenergic sympathetic features are connected to the central nervous system through neurons known as preganglionic sympathetic neurons, as is the case for jawed vertebrates. The anatomical targets of the sympathetic neurons in sea lampreys and their role during the life cycle of the fish also remain to be determined. In the context of development, it will be interesting to establish how the late acquisition of neuronal characteristics and proliferative activity in neural-crest-derived cells are regulated. This study underscores questions of how transcriptional circuits that are specific to autonomic neurons are established early in vertebrate development, how sympathetic circuitry became organized during vertebrate evolution, and how this circuitry might adapt to the physiological, in particular thermoregulatory, challenges that animals will face as a result of a changing climate in the future.

 Read the paper: Neural crest origin of sympathetic neurons at the dawn of vertebrates
Read the paper: Neural crest origin of sympathetic neurons at the dawn of vertebrates
 On the crest of becoming vertebrate
On the crest of becoming vertebrate
 Getting inside the oldest known vertebrate skull
Getting inside the oldest known vertebrate skull